
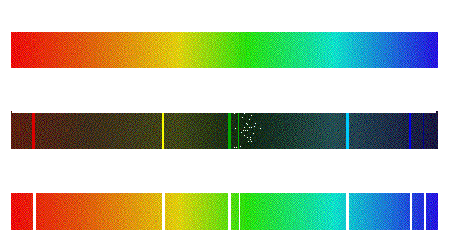
Create an account The Bohr Model and Atomic Spectra. The prominent mercury lines are at 435.835 nm (blue), 546.074 nm (green), and a pair at 576.959 nm and 579.065 nm (yellow-orange). Learn about the Bohr Model, atomic spectra, and how electrons emit different colors of light. The prominent mercury lines are at 435.835 nm (blue), 546.074 nm (green), and a pair at 576.959 nm and 579.065 nm (yellow-orange). However, when separated using a prism or diffraction grating, the. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Electrons in the gaseous atoms first become excited, and then fall back to lower energy levels, emitting light of a distinctive color in the process. At the right of the image are the spectral lines through a 600 line/mm diffraction grating. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.

However, hydrogen is able to emit multiple lines of color when voltage (energy) is added.

Such signs are excited by voltages of a few thousand volts produced by a transformer that raises the voltage of the ordinary AC line voltage.Īt left is a mercury spectral tube excited by means of a 5000 volt transformer. Contributors and Attributions Hydrogen has one energy level available when it is in the ground state. This is a section of the sign shown below, which has a central neon section and another gas mixture producing blue light around it. In 1885, a Swiss mathematics teacher, Johann Balmer (18251898), showed that the frequencies of the lines observed in the visible region of the spectrum of hydrogen fit a simple equation that can be expressed as follows: constant ( 1 22 1 n2) (7.3.1) (7.3. Then the image below was reduced and superimposed on the image above, because with the exposure reasonable for the bright tube, only the red lines were visible on the photograph. The image below is composed of segments of three photographs to make the yellow and green lines more visible along with the much brighter red lines. \) for a summary).This is an attempt to give a reasonable accurate picture of the appearance of the neon spectrum, but both the images are composite images.


 0 kommentar(er)
0 kommentar(er)
